Abstract
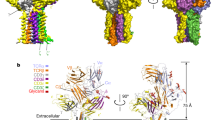
Gamma delta (γδ) T cells, a unique T cell subgroup, are crucial in various immune responses and immunopathology1-3. The γδ T cell receptor (TCR), generated by γδ T cells, recognizes a diverse range of antigens independently of the major histocompatibility complex2. The γδ TCR associates with CD3 subunits, initiating T cell activation and holding great potential in immunotherapy4. Here, we report the structures of two prototypical human Vγ9Vδ2 and Vγ5Vδ1 TCR–CD3 complexes5,6, unveiling two distinct assembly mechanisms that depend on Vγ usage. The Vγ9Vδ2 TCR–CD3 complex is monomeric, with considerable conformational flexibility in the TCRγ/TCRδ extracellular domain (ECD) and connecting peptides (CPs). The length of CPs regulates the ligand association and T cell activation. Additionally, a cholesterol-like molecule wedges into the transmembrane region, exerting an inhibitory role in TCR signaling. The Vγ5Vδ1 TCR–CD3 complex displays a dimeric architecture, where two protomers nestle back-to-back via their Vγ5 domains of TCR ECDs. Our biochemical and biophysical assays further corroborate the dimeric structure. Importantly, the dimeric form of the Vγ5Vδ1 TCR is essential for T cell activation. These findings reveal organizing principles of the γδ TCR–CD3 complex, providing insights into the γδ TCR unique properties and facilitating immunotherapeutic interventions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Figs 1–9 and Supplementary Table 1.
Rights and permissions
About this article
Cite this article
Xin, W., Huang, B., Chi, X. et al. Structures of human γδ T cell receptor–CD3 complex. Nature (2024). https://doi.org/10.1038/s41586-024-07439-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41586-024-07439-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.