August 10, 2022 report
Using a manganese polymer to separate xylene isomers
A team of researchers at Zhejiang University in China, working with colleagues at Rutgers University in the U.S., has developed a way to use a manganese polymer to separate xylene isomers. In their paper published in the journal Science, the group describes the process and notes that it is simpler and less expensive than other methods.
Xylene isomers are important chemical intermediates that are often used to make different types of plastics. Three of them are of particular value: par, meta and ortho. Unfortunately, as they are synthesized in standard processes, they come out bound together. In order to be useful, they must be separated. But doing so has proven to be time-consuming and expensive. This is because all three have similar structures and boiling points.
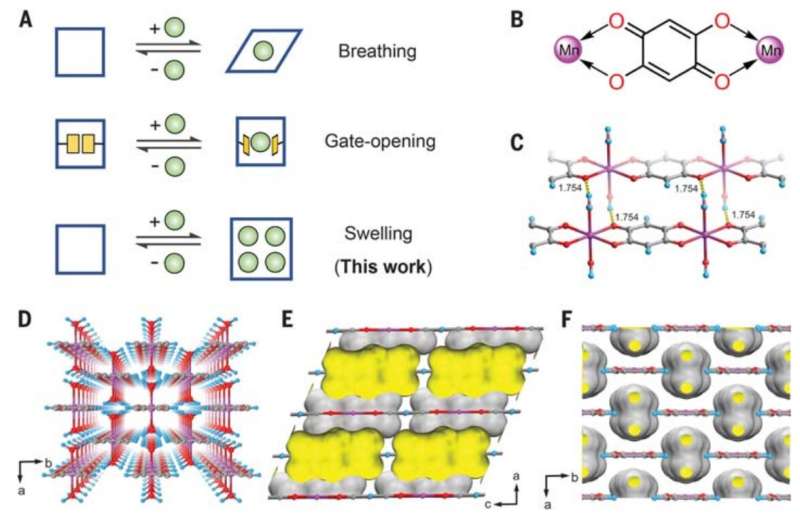
The work by the researchers involved finding a material that could serve as an adsorber—where molecules of a liquid or gas form a thin film on a surface that can then be collected. They searched for one-dimensional coordination porous polymers that were known to have flexibility and identified manganese, which initially seemed as if it would not work because its pores are too small. But the researchers found that when exposed to a xylene mixture, its structure swelled, increasing the distance between the polymer chains and making the pores bigger. And that allowed for adsorption and consequent separation of the isomers.
The researchers note that the pore size of manganese changed depending on temperature, so by applying differing temperatures to a given sample they could trap a desired isomer, isolating it from the others. They also note that the process works particularly well for isolating para-xylene, which is the most commonly used in making plastics. They believe their process should also be attractive to plastic makers because it avoids the use of distillation, which is notoriously dangerous. They conclude by claiming their process should be easy to scale, making it relevant for use in large scale operations.
More information: Liangying Li et al, Discrimination of xylene isomers in a stacked coordination polymer, Science (2022). DOI: 10.1126/science.abj7659
Journal information: Science
© 2022 Science X Network