This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists track 'doubling' in origin of cancer cells
Working with human breast and lung cells, Johns Hopkins Medicine scientists say they have charted a molecular pathway that can lure cells down a hazardous path of duplicating their genome too many times, a hallmark of cancer cells.
The findings, published in Science, reveal what goes wrong when a group of molecules and enzymes trigger and regulate what's known as the "cell cycle," the repetitive process of making new cells out of the cells' genetic material.
The findings could be used to develop therapies that interrupt snags in the cell cycle, and have the potential to stop the growth of cancers, the researchers suggest.
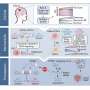
To replicate, cells follow an orderly routine that begins with making a copy of their entire genome, followed by separating the genome copies, and finally, dividing the replicated DNA evenly into two "daughter" cells.
Human cells have 23 pairs of each chromosome—half from the mother and half from the father, including the sex chromosomes X and Y—or 46 total, but cancer cells are known to go through an intermediate state that has double that number—92 chromosomes. How this happens was a mystery.
"An enduring question among scientists in the cancer field is: How do cancer cell genomes get so bad?" says Sergi Regot, Ph.D., associate professor of molecular biology and genetics at the Johns Hopkins University School of Medicine. "Our study challenges the fundamental knowledge of the cell cycle and makes us reevaluate our ideas about how the cycle is regulated."
Regot says cells that are stressed after copying the genome can enter a dormant, or senescent stage, and mistakenly run the risk of copying their genome again.
Generally and eventually, these dormant cells are swept away by the immune system after they are "recognized" as faulty. However, there are times, especially as humans age, when the immune system can't clear the cells. Left alone to meander in the body, the abnormal cells can replicate their genome again, shuffle the chromosomes at the next division, and a growing cancer begins.
In an effort to pin down details of the molecular pathway that goes awry in the cell cycle, Regot and graduate research assistant Connor McKenney, who led the Johns Hopkins team, focused on human cells that line breast ducts and lung tissue. The reason: These cells generally divide at a more rapid pace than other cells in the body, increasing the opportunities to visualize the cell cycle.
Regot's lab specializes in imaging individual cells, making it especially suited to spot the very small percentage of cells that don't enter the dormant stage and continue replicating their genome.
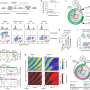
For this new study, the team scrutinized thousands of images of single cells as they went through cell division. The researchers developed glowing biosensors to tag cellular enzymes called cyclin dependent kinases (CDKs), known for their role in regulating the cell cycle.
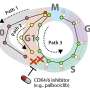
They saw that a variety of CDKs activated at different times during the cell cycle. After the cells were exposed to an environmental stressor, such as a drug that disrupts protein production, UV radiation or so-called osmotic stress (a sudden change in water pressure around cells), the researchers saw that CDK 4 and CDK 6 activity decreased.
Then, five to six hours later, when the cells started preparations to divide, CDK 2 was also inhibited. At that point, a protein complex called the anaphase promoting complex (APC) was activated during the phase just before the cell pulls apart and divides, a step called mitosis.
"In the stressed environment in the study, APC activation occurred before mitosis, when it's usually been known to activate only during mitosis," says Regot.
About 90% of breast and lung cells leave the cell cycle and enter a quiet state when exposed to any environmental stressors.
In their experimental cells, not all of the cells went quiet.
The research team watched as about 5% to 10% of the breast and lung cells returned to the cell cycle, dividing their chromosomes again.
Through another series of experiments, the team linked an increase in activity of so-called stress activated protein kinases to the small percentage of cells that skirt the quiet stage and continue to double their genome.
Regot says there are ongoing clinical trials testing DNA-damaging agents with drugs that block CDK. "It's possible that the combination of drugs may spur some cancer cells to duplicate their genome twice and generate the heterogeneity that ultimately confers drug resistance," says Regot.
"There may be drugs that can block APC from activating before mitosis to prevent cancer cells from replicating their genome twice and prevent tumor stage progression," says Regot.
Other researchers who contributed to the study include Yovel Lendner, Adler Guerrero-Zuniga, Niladri Sinha, Benjamin Veresko and Timothy Aikin from Johns Hopkins.
More information: Connor McKenney et al, CDK4/6 activity is required during G2 arrest to prevent stress-induced endoreplication, Science (2024). DOI: 10.1126/science.adi2421. www.science.org/doi/10.1126/science.adi2421