Cations found to be culprit behind degraded platinum electrodes

Electrochemical devices like batteries and fuel cells help power our modern lives. These devices traditionally contain a liquid electrolyte sandwiched between solid electrodes, and can generate electricity through chemical reactions, or alternatively, can undergo chemical reactions when subjected to an electrical current.
A rechargeable battery is a classic example of an electrochemical device that has both these functions. It is important to understand how electrochemical devices degrade during use if we want to increase their durability. However, the decay of the solid metal electrodes in electrochemical devices is poorly understood.
Now, a collaboration between Japanese, Korean, and American researchers is addressing this knowledge gap by investigating the degradation behavior of electrodes made of platinum—a stable noble metal like gold and silver—in different electrolyte solutions.
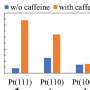
The researchers measured the degradation of platinum electrodes in electrolytes containing different cations, which are atoms with positive charges, from the same group of the periodic table (lithium, sodium, potassium, and cesium). Their results revealed that the cation identity affected platinum degradation: larger cations suppressed platinum dissolution compared with that in systems with smaller cations.
"We monitored platinum dissolution in real time," says lead author Haesol Kim. "Our results revealed that platinum leaching decreased as the atomic number (and size) of the cation increased. That is, electrode degradation is influenced by a cation effect."

Intrigued by this effect, the researchers conducted further experiments and computer simulations to identify the origin of this behavior.
"Our computational simulations indicated that the hydroxide ions near the platinum electrode strongly affected platinum leaching," explains senior author Chang Hyuck Choi. "Hydroxide ions promoted the diffusion of platinum ions into the bulk electrolyte by weakening the force for platinum redeposition on the electrode surface."
The negatively charged hydroxide ions acted as a shield around the positively charged platinum ions, helping them to move away from the electrode surface. But how did this relate to the cation effect?
The cations in the electrolyte influenced the concentration of hydroxide ions near the platinum electrode surface. Cations with higher acidity provided a higher hydroxide ion concentration near the platinum surface than cations with lower acidity. In the investigated cation series, the smaller cations had higher acidity than the larger cations. As a result, platinum electrode degradation was accelerated in the presence of smaller cations.
The finding that electrode stability is directly affected by the type of cations in the electrolyte is an exciting finding and will give researchers an area to focus on in their quest to create the durable electrochemical devices needed to power the future.
More information: Haesol Kim et al, Cation Effect on the Electrochemical Platinum Dissolution, Journal of the American Chemical Society (2025). DOI: 10.1021/jacs.4c17833
Journal information: Journal of the American Chemical Society
Provided by Osaka University